
Publication
Article
Pharmacy Practice in Focus: Oncology
Navigating Drug Resistance in Chronic Myelocytic Leukemia
Author(s):
Opportunities for further optimization of CML management remain.
Chronic Myelocytic Leukemia (CML) arises from abnormal pluripotent hematopoietic progenitor cells promoting excessive production of immature cells in the myeloid lineage, resulting in a hypercellular bone marrow and symptoms such as splenomegaly. In the United States, there are an estimated 8860 new cases annually, with a slightly greater incidence of the disease among men than women. In 2021, 15% of all adult leukemia cases were CML cases, with a median age of patients at diagnosis between 64 and 67 years.1,2
In more than 95% of patients with CML, the excessive production of immature, leukemic cells have a hallmark Philadelphia chromosome (Ph) due to a reciprocal translocation between chromosomes 9 and 22, resulting in the fusion tyrosine kinase BCR-ABL1. RNA expression of BCR-ABL1 is monitored by reverse transcription–quantitative polymerase chain reaction to determine the efficacy of tyrosine kinase inhibitors (TKIs), a mainstay of CML treatment.1,3
CML is subdivided into phases, in part by the percentage of myeloblasts in peripheral blood. Most patients are diagnosed with chronic phase CML (CML-CP); however, additional CML diagnoses include the more advanced accelerated phase (CML-AP) and blast phase (CML-BP).
In unmanaged CML, the disease is expected to progress to CML-BP within 3 to 8 years.4 Although TKIs play a role in all phases of disease, the phase of the disease at diagnosis will influ-ence the initial selection of TKI. For instance, second- or later-generation TKIs are preferred for CML-AP, and TKIs are often combined with intensive chemotherapy for CML-BP. However, a common thread among all phases is the significance of TKI adherence, as data have demonstrated that nonadherence is associated with reduced survival.5
The goal of TKI therapy is to attain complete cytogenetic response (CCyR), signifying no detectable Ph-positive cells in the bone marrow—equivalent to BCR-ABL1 at approximately 1% on the International Scale—in 12 months or earlier following the initiation of therapy.1,6 Attainment of CCyR is associated with low rates of progres-sion to CML-AP and CML-BP, as well as a high rate of overall survival. However, failure to meet BCR-ABL1 thresholds at 3, 6, and 12 months into TKI therapy is associated with less durable response, such as higher risk of cytogenetic relapse, and may be indicative of resistance to the TKI. In such cases of potential TKI resistance, the consideration of an alternate agent is strongly recommended.1
Primary Resistance
Primary resistance, or refractoriness, is characterized by an inability to attain time-dependent end points or other end points of response, such as CCyR, upon initiation of TKI therapy.8-10 Several BCR-ABL1–independent mechanisms— including drug transporters and other pharmacokinetic considerations, such as signaling pathway activation and epigenetic dysregulation—have been implicated as contributing factors to primary resistance. However, these targets vary in terms of druggability, and assessment of these mechanisms (with the exception of drug-drug interactions) is generally not pursued during therapeutic planning.1,8,9,11-14
Adenosine triphosphate (ATP)-binding cassette (ABC) transporters include P glycoprotein (P-gp) and breast cancer resistance protein (BCRP), which are present in primitive normal hematopoietic stem cells, as well as the liver, kidney, and other organs.15 Over-expression of these ABC transporters, due to single nucleotide polymorphisms or other causes, promotes active TKI efflux and decrease in intracellular drug accumulation, with the end result being resistance to imatinib (Gleevec; Novartis).9,16,17
Imatinib dose escalation to address overactive efflux transporters is controversial.9 Combining imatinib with some ABC inhibitors (or chemosensitizers), such as verapamil and valspodar, has historically been poorly tolerated because of the off-target effects of these inhibitors. However, elacridar, a dual P-gp and BCRP inhibitor, in combination with imatinib, has demon-strated promising in vitro efficacy for overcoming resistance to imatinib. Further investigation is required before concomitant chemosensitizers can be applied in clinical practice.18
Polymorphisms of the cellular influx–modulating organic cation transporter (OCT1) have also been proposed as an important factor regulating intracellular imatinib availability.15,19 Low OCT1 activity has been associated with long-term imatinib resistance in CML vs the association of high OCT1 activity with improved major molecular response (MMR) rates.20 However, the clinical applications of the association between low OCT1 activity and imatinib resistance remain unclear, in part due to inconsistent find-ings for specific OCT1 variants impacting imatinib response.13,19,21 Furthermore, the impact of concomitant medications should be assessed to prevent suboptimal TKI concen-trations and responses. Extensive hepatic first pass metabolism characterizes TKI metabolism; because of this, a current medication list should be assessed for medications and supplements inducing cytochrome P450 (CYP) 3A4 prior to TKI initiation. Depending on the TKI, a dose modification or complete avoidance of strong CYP3A4 inhibitors or inducers may be recommended by prescribing information.21,22 Manufacturer guidance should also be referenced regarding the allowance of—or any dosing interval required between—administrations of TKIs and acid-suppressing agents such as proton pump inhibitors, H2-receptor antagonists, and antacids. Certain TKIs, such as dasatinib (Sprycel; Bristol Myers Squibb), have poor solubility in acid-suppressed environ-ments.22
Other mechanisms recognized as having a relatively larger impact on BCR ABL–independent resistance include a bone marrow niche impeding TKI action on CML leukemic stem cells (LSCs), which is marked by CD26 (otherwise known as dipeptidyl peptidase IV/DPP4) and promotion of residual disease; the sustained CML LSC maintenance due to JAK/STAT signaling that promotes signaling for cell growth, invasion, and inflammation; and expansion/self renewal of CML LSCs due to deregulation of the Wnt/β-catenin pathway.23,24 Various trials have attempted to address these factors affecting BCR-ABL independent resis-tance by adding targeted agents to TKI therapy. To date, trialed targeted agents include DPP4 inhibitor vildagliptin, JAK inhibitor ruxolitinib, and Wnt inhibi-tion via a porcupine acyl transferase inhibitor.23-25
Additionally, epigenetic modifications, such as histone acetylation and DNA methylation, contribute to CML LSC persistence and TKI resistance. Further, many targeted agents are under investigation, including histone deacetylase (HDAC) inhibitors—such as pracinostat (SB939), vorinostat (Zolinza; Merck), and panobinostat (Farydak; Secura Bio, Inc)—and sirtuin, or class III HDAC inhibitors such as nicotinamide and tenovin-6.26,2
Secondary Resistance
Secondary resistance, or acquired resistance, is character-ized by the initial achievement of response to a TKI that is followed by its loss.8-10 The mechanisms of secondary resistance most commonly encompass factors dependent on BCR-ABL, although BCR-ABL–independent mecha-nisms may also contribute to secondary resistance.9,14
Emerging BCR-ABL point mutations, ascertained by a BCR-ABL1 mutation analysis via traditional Sanger sequencing or more sensitive next-generation sequencing, are a major contributor to secondary resistance and treatment failure, with the presence of certain mutations dictating the selection of a second- or subsequent-line TKI (Table).1,28 Notable sites of BCR-ABL point mutations are at the P-loop (ATP-binding site), TKI contact site (affecting TKI binding), C-loop (catalytic domain), A-loop (activation loop), and the myristate pocket.18
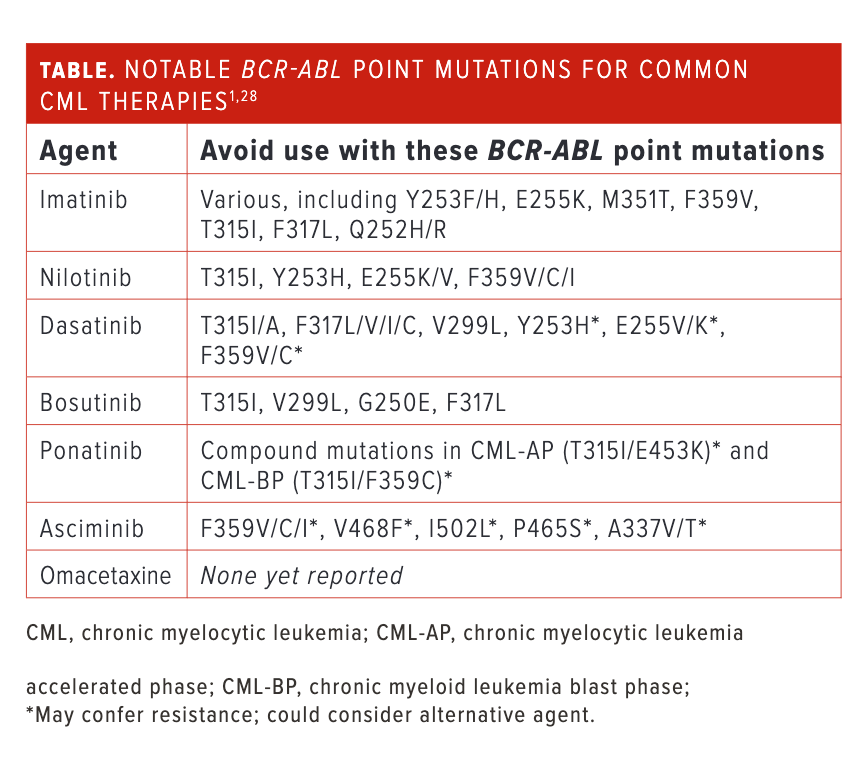
One point mutation of interest is T315I, which is found in approximately 13% of patients with CML who experience imatinib resistance.29 This mutation confers resistance to most FDA-approved TKIs that are competitive at the ATP-binding site. One excep-tion is the third-generation TKI ponatinib (Iclusig; Takeda), with a carbon-carbon triple bond extending from the purine scaffold that minimizes the impact of steric hindrance caused by the T315I amino acid substitution.30 Another option in the setting of T315I mutation is omacetaxine (Synribo; Cephalon, Inc), which inhibits protein synthesis by binding to the A-loop, effectively reducing supply of BCR-ABL and other proteins with short half-lives.31,32
The final commercially available option is asciminib (Scemblix; Novartis), which acts as a selective allo-steric inhibitor by binding to the myristoyl pocket of BCR-ABL1 kinase domain.33 As with the other agents, asciminib is not immune to non-T315I BCL-ABLmutations. There are concerns about resistance to asciminib, with emerging mutations in the myristoyl-binding domain, such as V468F, I502L, P465S, and A337V/T, as well as mutations at codon 359 (F359V/C/I) near the C-loop.34
There are also agents in the pipeline that aim to provide additional options for T315I and other point mutations. Olverembatinib (HQP1351; Ascentage Pharma) is a third-generation TKI competitive at the ATP-binding site. Patients take 40 mg orally every other day. Two single-arm, open-label phase 2 trials are investigating olverembatinib, with patients with TKI resistance (T315I-mutated) enrolled into either the CML-CP trial (NCT03883087) or the CML-AP trial (NCT03883100), based on their diagnosis. During the trials, MMR was achieved in 56.1% of patients with CML-CP and 39.1% of patients with CML-AP.35 Additionally, investigators observed grade 3 or higher adverse events (AEs) at more than 10% incidence, including hematologic toxicities in both CML-CP and CML-AP cohorts, with grade 3 or higher elevated creatine kinase found to be common among patients with CML-CP.35,36
Also in the pipeline, vodobatinib (K0706; Sun Pharma), a third-generation TKI, was demonstrated to be ineffective against the T315I mutation, but it may confer other benefits with other settings of resistant CML. This agent was examined in an open-label, phase 1/2 trial (NCT02629692) in a patient population with CML/Ph-positive acute lymphoblastic leukemia to determine efficacy for those who are heavily pretreated (3 or more TKIs failed) and/or with comorbidities precluding use of nilotinib (Tasigna; Novartis), dasatinib, and ponatinib. During this trial, MMR was achieved for 47% of patient groups that included both ponatinib-naïve and postponatinib CML-CP and for 33% of patients (1 of 3 patients) with CML-AP. Additionally, investigators observed grade 3 or higher AEs that included hematologic toxicities, increased amylase/lipase levels, congestive cardiac failure, hypertension, amnesia, and fatal intracranial hemorrhage.37
PF-114 is a fourth-generation TKI with a ponatinib-like structure but without VEGF inhibition.33 A phase 1/2 trial (NCT02885766) enrolled a CML-CP and CML-AP patient population, 31.4% of whom had T315I mutation. The patient population was also notable for being heavily pretreated, as 49% had received 3 or more prior TKIs.38 At a dose range of 50 to 600 mg, MMR was attained in 6.3% of patients with a T315I mutation. Additionally, although the toxicities were not extensively reported, grade 3 psoriasis-like skin lesions were noted as the dose-limiting toxicity.38,39
Furthermore, BCR-ABL–independent mechanisms of secondary resistance are garnering interest as potential druggable targets, with the caveat that phase 2 data remain to be seen for most of these agents, including those addressed above. Additionally, aurora kinase inhibitors, such as tozasertib (VX-680; Vertex Pharmaceuticals), danusertib (PHA-739358; Selleck), and alisertib (MLN8237; Takeda) dysregulate cell division and may be effective as a subsequent-line therapy with T315I mutation.40
Investigational agents targeting the RAS/MEK/ERK pathway include inhibitors of farnesyltransferase, such as tipifarnib (Zarnestra; Johnson & Johnson) and lonafarnib (Zokinvy; Eiger BioPharmaceuticals, Inc), which downregulate RAS activation; MEK, such as selumetinib (Koselugo; Alexion Pharmaceuticals, Inc) and trametinib (Mekinist; Novartis); and PKC, such as enzastaurin (DB102; Denovo Biopharma LLC). The PI3K/AKT/mTOR pathway is also under active examination with inhibitors of PI3K, such as pictilisib (GDC-0941; Genentech Inc); mTOR, such as sirolimus (Rapamune; Pfizer Inc) and everolimus (Afinitor; Novartis); and AKT, such as MK-2206.18 Addition-ally, BCL2 inhibition, using agents such as sabutoclax (BI-97C1; Selleck), obatoclax (GX15-070; Teva Pharmaceuticals), and venetoclax (Venclexta; AbbVie and Genentech), has been implicated in reduction of quiescent CML LSCs.27
TKIs have transformed the treatment landscape of CML and dramatically improved overall survival with newly diagnosed CML, with the estimated 8-year survival rate up from 6% before 1975 to 87% since the advent of imatinib in 2001.40 However, opportuni-ties for continued optimization of CML management remain with numerous mechanisms of primary and secondary resistance to TKIs under investigation.
References
- NCCN. Clinical Practices Guidelines in Oncology. Chronic myeloid leukemia, version 3.2022. Accessed May 20, 2022. https://www.nccn.org/guidelines/guidelines-process/transparency-process-and-recommendations/GetFileFromFileManager?fileManagerId=11492
- Key statistics for chronic myeloid leukemia. American Cancer Society. Updated January 12, 2022. Accessed May 20, 2022. https://www.cancer.org/cancer/chronic-myeloid-leukemia/about/statistics.html
- Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. 2020;95(6):691-709. doi:10.1002/ajh.25792
- Saxena K, Jabbour E, Issa G, et al. Impact of frontline treatment approach on outcomes of myeloid blast phase CML. J Hematol Oncol. 2021;14(1):94. doi:10.1186/s13045-021-01106-1
- Tan BK, Bee PC, Chua SS, Chen LC.Monitoring and improving adherence to tyrosine kinase inhibitors in patients with chronic myeloid leukemia: a systematic review. Patient Prefer Adherence. 2021;15:2563-2575.doi:10.2147/PPA.S269355
- Radin DP, Smith G, Moushiaveshi V, Wolf A, Bases R, Tsirka SE.Lucanthone targets lysosomes to perturb glioma proliferation, chemoresistance and stemness, and slows tumor growth in vivo. Front Oncol. 2022;12:852940. doi:10.3389/fonc.2022.852940
- Shaya J, Pettit K, Kandarpa M, Bixby D, Mercer J, Talpaz M. Late responses in patients with chronic myeloid leukemia initially refractory to tyrosine kinase inhibitors. Clin Lymphoma Myeloma Leuk. 2022;22(1):17-23. doi:10.1016/j.clml.2021.07.001
- Pietarinen PO, Pemovska T, Kontro M, et al.Novel drug candidates for blast phase chronic myeloid leukemia from high-throughput drug sensitivity and resistance testing. Blood Cancer J. 2015;5(5):e309. doi:10.1038/bcj.2015.30
- Quintás-Cardama A, Kantarjian HM, Cortes JE.Mechanisms of primary and secondary resistance to imatinib in chronic myeloid leukemia. Cancer Control. 2009;16(2):122-131. doi:10.1177/107327480901600204
- Patel AB, O’Hare T, Deininger MW. Mechanisms of resistance to ABL kinase inhibition in chronic myeloid leukemia and the development of next generation ABL kinase inhibitors. Hematol Oncol Clin North Am. 2017;31(4):589-612. doi:10.1016/j.hoc.2017.04.007
- Bavaro L, Martelli M, Cavo M, Soverini S.Mechanisms of disease progression and resistance to tyrosine kinase inhibitor therapy in chronic myeloid leukemia: an update. Int J Mol Sci. 2019;20(24):6141. doi:10.3390/ijms20246141
- De Santis S, Monaldi C, Mancini M, Bruno S, Cavo M, Soverini S.Overcoming resistance to kinase inhibitors: the paradigm of chronic myeloid leukemia. Onco Targets Ther. 2022;15:103-116. doi:10.2147/OTT.S289306
- Loscocco F, Visani G, Galimberti S, Curti A, Isidori A.BCR-ABL independent mechanisms of resistance in chronic myeloid leukemia. Front Oncol. 2019;9:939.doi:10.3389/fonc.2019.00939
- Choudhuri S, Klaassen CD. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int J Toxicol. 2006;25(4):231-259. doi:10.1080/10915810600746023
- Nakanishi T, Shiozawa K, Hassel BA, Ross DD. Complex interaction of BCRP/ABCG2 and imatinib in BCR-ABL-expressing cells: BCRP-mediated resistance to imatinib is attenuated by imatinib-induced reduction of BCRP expression. Blood. 2006;108(2):678-684. doi:10.1182/blood-2005-10-4020
- Maia RC, Vasconcelos FC, Souza PS, Rumjanek VM.Towards comprehension of the ABCB1/P-glycoprotein role in chronic myeloid leukemia. Molecules. 2018;23(1):119.doi:10.3390/molecules23010119
- Alves R, Gonçalves AC, Jorge J, Almeida AM, Sarmento-Ribeiro AB.Combination of elacridar with imatinib modulates resistance associated with drug efflux transporters in chronic myeloid leukemia. Biomedicines. 2022;10(5):1158. doi:10.3390/biomedicines10051158
- Watkins DB, Hughes TP, White DL. OCT1 and imatinib transport in CML: is it clinically relevant? Leukemia. 2015;29(10):1960-1969. doi:10.1038/leu.2015.170
- Guilhot F, Hughes TP, Cortes J, et al. Plasma exposure of imatinib and its correlation with clinical response in the tyrosine kinase inhibitor optimization and selectivity trial. Haematologica. 2012;97(5):731-738. doi:10.3324/haematol.2011.045666
- Kaehler M, Cascorbi I. Pharmacogenomics of impaired tyrosine kinase inhibitor response: lessons learned from chronic myelogenous leukemia. Front Pharmacol. 2021;12:696960. doi:10.3389/fphar.2021.696960
- Sprycel. Prescribing information. Bristol Myers Squibb; 2021. Accessed May 20, 2022. https://packageinserts.bms.com/pi/pi_sprycel.pdf
- Sicuranza A, Raspadori D, Bocchia M.CD26/DPP-4 in chronic myeloid leukemia. Cancers (Basel). 2022;14(4):891. doi:10.3390/cancers14040891
- Houshmand M, Simonetti G, Circosta P, et al. Chronic myeloid leukemia stem cells. Leukemia. 2019;33(7):1543-1556. doi:10.1038/s41375-019-0490-0
- Mojtahedi H, Yazdanpanah N, Rezaei N. Chronic myeloid leukemia stem cells: targeting therapeutic implications. Stem Cell Res Ther. 2021;12(1):603. doi:10.1186/s13287-021-02659-1
- Bugler J, Kinstrie R, Scott MT, Vetrie D. Epigenetic reprogramming and emerging epigenetic therapies in CML. Front Cell Dev Biol. 2019;7:136. doi:10.3389/fcell.2019.00136
- Massimino M, Stella S, Tirrò E, et al. Non ABL-directed inhibitors as alternative treatment strategies for chronic myeloid leukemia. Mol Cancer. 2018;17(1):56. doi:10.1186/s12943-018-0805-1
- Andretta E, Costa C, Longobardi C, et al.Potential approaches versus approved or developing chronic myeloid leukemia therapy. Front Oncol. 2021;11:801779.doi:10.3389/fonc.2021.801779
- Ursan ID, Jiang R, Pickard EM,Lee TA, Ng D, Pickard AS. Emergence of BCR-ABL kinase domain mutations associated with newly diagnosed chronic myeloid leukemia: a meta-analysis of clinical trials of tyrosine kinase inhibitors. J Manag Care Spec Pharm. 2015;21(2):114-122. doi:10.18553/jmcsp.2015.21.2.114
- Tan FH, Putoczki TL, Stylli SS, Luwor RB.Ponatinib: a novel multi-tyrosine kinase inhibitor against human malignancies. Onco Targets Ther. 2019;12:635-645. doi:10.2147/OTT.S189391
- Gandhi V, Plunkett W, Cortes J. Omacetaxine: a protein translation inhibitor for treatment of chronic myelogenous leukemia. Clin Cancer Res. 2014;20(7):1735-1740. doi:10.1158/1078-0432.CCR-13-1283
- Winer ES, DeAngelo DJ. A review of omacetaxine: a chronic myeloid leukemia treatment resurrected. Oncol Ther. 2018;6(1):9-20. doi:10.1007/s40487-018-0058-6
- Hughes TP, Mauro MJ, Cortes JE, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. 2019;381(24):2315-2326. doi:10.1056/NEJMoa1902328
- Eide CA, Zabriskie MS, Savage Stevens SL, et al.Combining the allosteric inhibitor asciminib with ponatinib suppresses emergence of and restores efficacy against highly resistant BCR-ABL1 mutants. Cancer Cell. 2019;36(4):431-443.e5. doi:10.1016/j.ccell.2019.08.004
- Jiang Q, Huang X, Chen Z, et al. Updated results of pivotal phase 2 trials of olverembatinib (HQP1351) in patients (pts) with tyrosine kinase inhibitor (TKI)-resistant BCR-ABL1T315I-mutated chronic- and accelerated-phase chronic myeloid leukemia (CML-CP and CML-AP). Poster presented at: 63rd American Society of Hematology Annual Meeting & Exposition; December 11-14, 2021; Atlanta, GA.[YQ1]
- Qian J, Shi D, Li Z, et al. Updated safety and efficacy results of phase 1 study of olverembatinib (HQP1351), a novel third-generation BCR-ABL tyrosine kinase inhibitor (TKI), in patients with TKI-resistant chronic myeloid leukemia (CML). Blood. 2021;138(suppl 1):311. doi:10.1182/blood-2021-153065
- Cortes JE, Saikia T, Kim D, et al. Phase 1 trial of vodobatinib, a novel oral BCR-ABL1 tyrosine kinase inhibitor (TKI): activity in CML chronic phase patients failing TKI therapies including ponatinib. Poster presented at: 63rd American Society of Hematology Annual Meeting & Exposition; December 11-14, 2021; Atlanta, GA.[YQ2]
- Turkina AG, Vinogradova O, Lomaia E, et al. PF-114: a 4th generation tyrosine kinase-inhibitor for chronic phase chronic myeloid leukaemia including BCRABL1T315I. Blood. 2019;134(suppl 1):1638. doi:10.1182/blood-2019-127951
- Turkina AG, Vinogradova O, Lomaia E, et al. Phase-1 study of PF-114 mesylate in CML failing prior tyrosine kinase-inhibitor therapy. Blood. 2018;132(suppl 1):790. doi:10.1182/blood-2018-99-116803
- Cortes J, Lang F. Third-line therapy for chronic myeloid leukemia: current status and future directions. J Hematol Oncol. 2021;14(1):44. doi:10.1186/s13045-021-01055-9
- Kantarjian H, O’Brien S, Jabbour E, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119(9):1981-1987. doi:10.1182/blood-2011-08-358135
About the Author
Grace Baek, PharmD, BCOP, is a clinical hematology/oncology pharmacist in the Department of Pharmacy at Fred Hutchinson Cancer Center and the Department of Pharmacy at the University of Washington School of Medicinein Seattle.

Newsletter
Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.




