News
Article
Pharmacists Will Play an Essential Role in Optimizing Vutrisiran Therapy for ATTR-CM
Author(s):
Key Takeaways
- ATTR-CM is a potentially fatal disease causing amyloid deposits in the heart, leading to cardiomyopathy and heart failure.
- Vutrisiran, an RNA interference therapeutic, targets the main cause of ATTR-CM by reducing transthyretin production.
If approved, vutrisiran would be the first FDA-approved therapy to treat both the polyneuropathy and cardiomyopathy aspects of transthyretin amyloidosis (ATTR).
Complex treatment options are a major barrier to care for patients with transthyretin amyloid cardiomyopathy (ATTR-CM), making clinical pharmacists crucial for patient education and navigating the therapeutic landscape.1 However, vutrisiran (Amvuttra; Alnylam Pharmaceuticals, Inc.), an investigational RNA interference therapeutic in development for ATTR-CM, could further treatment options.2
Image credit: ckybe | stock.adobe.com
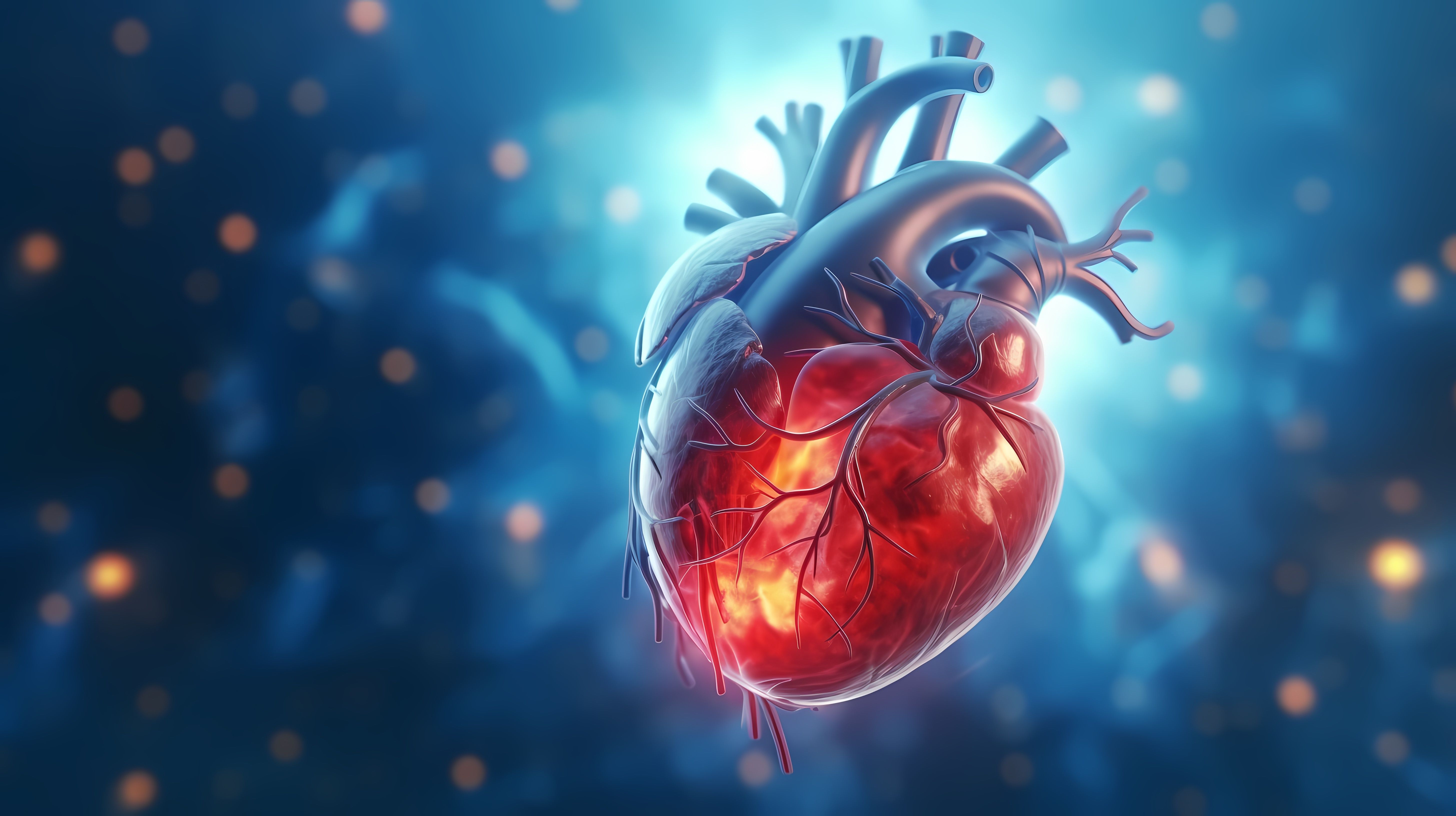
ATTR-CM is a potentially fatal disease of the heart muscle, causing the transthyretin protein to misshapen and build up in the heart, nerves, and other organs. Amyloid deposits stiffen the heart walls, preventing the left ventricle from properly filling with blood, a condition known as cardiomyopathy. This stiffness can progress to impaired pumping and ultimately heart failure (HF). ATTR-CM is often underdiagnosed in patients.3
ATTR-CM symptoms are often subtle and varied, leading to frequent misdiagnosis, as early stages can resemble other cardiac conditions like hypertensive HF or hypertrophic cardiomyopathy. Some individuals may be asymptomatic, while others progress to end-stage heart failure.3
According to the American Heart Association, ATTR-CM has two forms: hereditary (hATTR-CM) and wild-type (wATTR-CM). Symptoms of hATTR-CM can present as early as 30 years of age; however, they often occur later in life. Risk factors for hATTR-CM include having a family member with ATTR-CM or HF, being aged 50 and older, Black, and/or male. Unlike hATTR-CM, wATTR-CM does not run in families and commonly impacts the heart and causes peripheral neuropathy. Adults aged 65 years and older and/or men face an increased risk of developing wATTR-CM.3
As a subcutaneously administered RNA interference therapeutic agent, vutrisiran inhibits the production of hepatic transthyretin.4 Vutrisiran works by quickly reducing both mutant and wild-type transthyretin, targeting the main cause of ATTR-CM. If approved, vutrisiran would be the first FDA-approved therapy to treat both the polyneuropathy and cardiomyopathy aspects of ATTR.2
Previous research published in The New England Journal of Medicine demonstrated that vutrisiran treatment led to a lower risk of death from any cause and recurrent cardiovascular events, compared to a placebo among individuals with ATTR-CM. A total of 655 individuals were included in the double-blind, randomized trial to receive a 25 mg dose of vutrisiran (326) or placebo (329) every 12 weeks for up to 36 months.4
The study's primary end point was to assess a combined outcome of death from any cause and repeated cardiovascular events. Secondary end points included evaluating overall death rates, changes in walking distance over 6 minutes, and changes in patient-reported quality of life as measured by the Kansas City Cardiomyopathy Questionnaire-Overall Summary (KCCQ-OS) score.4
The results demonstrated that vutrisiran significantly reduced the risk of death and cardiovascular events compared to placebo, with a hazard ratio of 0.72 (P = 0.01) in the overall population and 0.67 (P = 0.02) in the monotherapy group. Specifically, 125 vutrisiran patients and 159 placebo patients experienced primary end point events. Further results displayed that vutrisiran decreased the risk of death from any cause over 42 months, with a hazard ratio of 0.65 (P = 0.01). Additionally, patients treated with vutrisiran showed a 26.5-meter improvement in the 6-minute walk test and a 5.8-point improvement in the KCCQ-OS score compared to placebo.4
Adverse event rates were similar between groups, with 99% in the vutrisiran group and 98% in the placebo group experiencing any adverse event, and serious adverse events occurring in 62% and 67%, respectively.4
The FDA accepted for review a supplemental new drug application for vutrisiran in November 2024, indicated for the treatment of ATTR-CM. The drug has a Prescription Drug User Fee Act (PDUFA) date set for March 23, 2025.2
Despite the upcoming approval, the pharmacist’s role in medication selection and education remains crucial for patients with ATTR-CM. The pharmacist’s responsibility also includes explaining medication benefits, overseeing treatment acquisition, and identifying financial assistance and alternative therapies to optimize treatment outcomes.1






