News
Article
Harnessing the Tumor Microenvironment for Enhanced Cancer Treatment
Author(s):
Key Takeaways
- The TME's complexity involves pro-tumor forces like M2 macrophages and regulatory T-cells that suppress immune responses and promote tumor growth.
- Anti-tumor forces, including CD8+ T-cells and NK cells, play crucial roles in combating tumor proliferation through cytotoxic mechanisms.
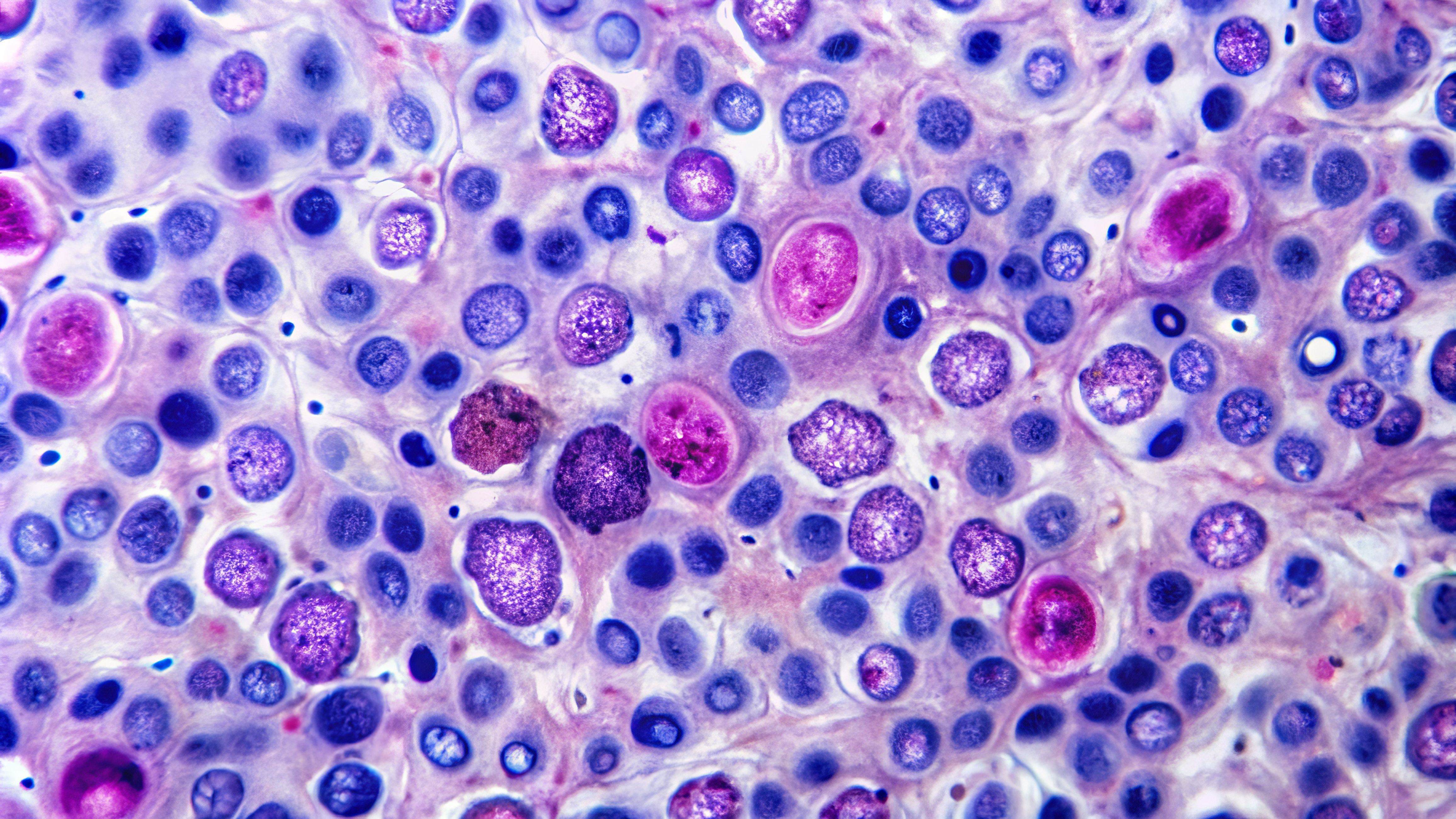
The tumor microenvironment (TME) is a heterogeneous and dynamic ecosystem where immune cells and tumor cells interact in ways that either promote or suppress cancer progression. At the 2025 ASCO Annual Meeting, researchers emphasized how the immune system’s role within the TME is shaped by this complexity, highlighting the roles of both pro-tumor and anti-tumor forces that influence therapeutic outcomes. Their insights reveal how the TME can be manipulated to improve immunotherapy responses.
T-cells attacking cancer cells | Image Credit: © HISTOCK - stock.adobe.com

“The [TME] is complex,” explained Judith A. Varner, PhD, BS, from the University of California San Diego. “On the one hand, we have pro-tumor forces in which a number of immune cell types, such as pro-tumor macrophages, regulatory B-cells, CD4+ T-cells, known as regulatory T-cells [Tregs], and fibroblasts that produce collagen, all work together to stimulate tumor growth and metastasis.”1
Macrophages are of particular interest due to their prevalence, comprising 30% to 60% of the tumor mass in some cancers. They are differentiated into 2 subgroups: M1, which are “classically activated” and act as antitumoral agents; and M2, which are “alternatively activated” and contain tumor-associated macrophages that facilitate progression through immunosuppression. Macrophages are typically in the anti-inflammatory M2 state, which suppresses T-cell function and creates an environment that prevents them from effectively attacking tumor cells.1,2
"Macrophages play essential roles in embryonic development as they are important in laying down branching networks of blood vessels and other branching tissues, and yet, they're also important in combating infectious diseases by recognizing and targeting pathogens,” said Varner.1
Macrophages create barriers that prevent T-cells from attacking tumors through various mechanisms and in conjunction with other pro-tumor forces, such as fibroblasts. Fibroblasts produce collagen that forms a dense network around tumors, creating barriers that physically block T-cells from entering tumor areas. Macrophages help fibroblasts produce collagen, reinforcing physical barriers that block T-cell infiltration.1
On their own, macrophages can also prevent T-cells from entering tumors through spatial positioning. In some tumors, T-cells are "excluded" from the tumor core and macrophages position themselves strategically to prevent penetration. For example, in head and neck tumors, T-cells were observed surrounding the tumor but were unable to enter.1
Other pro-tumor forces drive tumor progression by interacting with the immune system, such as regulatory B cells and Tregs. These cell types contribute to immune suppression as well as suppression of immune system activation. They create environments that protect tumor cells and prevent effective anti-tumor immune responses.1
Anti-tumor forces are critical for combatting tumor cell proliferation through direct cytotoxic mechanisms. CD8+ T cells kill tumor cells by forming synapses and releasing enzymes like perforin and granzymes that break down their contents. Effector cytolytic T-cells operate similarly, producing enzymes that destroy cancer cells as part of an active immune assault. Natural killer (NK) cells also contribute to this anti-tumor response by working alongside T-cells to identify and eliminate malignant cells through similar cytotoxic pathways.1
Pro-inflammatory M1 macrophages express lytic factors and play a dual role recognizing pathogens and signaling the immune system by attracting T cells, B cells, and other immune effectors to the tumor site. Activated dendritic cells are also essential, as they present tumor antigens to T-cells and help initiate immune responses. Additionally, dendritic cells ensure a more targeted and sustained attack against tumor cells by priming and arming CD8+ T-cells. Together, these immune cells form a coordinated defense network that directly attacks cancer cells and orchestrates a broader anti-tumor immune response.1
Interferons are cytokines that are released in the presence of pathogens in acute and chronic infections, as well as in the TME. In the TME, interferons have context-dependent effects and can be both beneficial and detrimental to immune system responses to tumor cells. In acute Infections, interferons promote initial T-cell priming and generate effector and memory populations to help eliminate pathogens quickly. In chronic infections, they drive T-cell exhaustion and regulate and control immune response to prevent overactive or misdirected immune attacks. Chronic interferon can drive T-cell exhaustion, as well as inflammatory memory in cancer cells.1
“Aberrant nucleic acids—whether it's the DNA from micronuclei or the double-stranded RNA generated by these endogenous and derepressed retroviruses—can activate the same type of pattern recognition receptors or sensors that are present in the cytosol that normally engage viral RNA or viral DNA,” explained Andy J. Minn, MD, PhD, from the University of Pennsylvania. “So, this leads to the generation of interferon and then activates JAK [Janus kinase] stat signaling and the induction of hundreds of genes that are known as interferon-stimulated genes, or ISGs, many of which have direct antiviral effects or effects on the immune system.”1
Like interferon, some ISGs are "good" and predict positive responses, are associated with better immunotherapy outcomes, and enhance initial immune activation. Alternatively, "bad" ISGs predict poor treatment outcomes, are associated with tumor relapse and T-cell exhaustion, and promote immune evasion. “Bad” ISGs help cancer cells mimic chronic viral infections to create feed-forward loops that reinforce suppressive tumor immune states.1
“What we believe happens is that cancer cells, when they're exposed to chronic levels of interferon, in this case, interferon gamma, that leads to the cancer cells adapting or undergoing epigenetic reprogramming, very similar to inflammatory memory,” said Minn.1
Researchers are exploring JAK inhibitors to block chronic interferon signaling with the goal of resetting the tumor immune system state to prevent T-cell exhaustion and improve immunotherapy responses. One specific JAK inhibitor discussed was itacitinib (Incyte Corporation), a JAK1-selective inhibitor evaluated in patients with non-small cell lung cancer.1
In the study conducted by Minn and his colleagues, itacitinib was administered with pembrolizumab (Keytruda; Merck & Co). The approach was carefully designed to target chronic interferon signaling and preserve the beneficial acute interferon response while selectively disrupting the chronic signaling that undermines immune effectiveness. The study design contributed to notable clinical improvements, including an overall response rate of 67% and a marked improvement in overall survival. The researchers observed that the addition of the JAK inhibitor appeared to reset T-cell differentiation, steering it away from exhaustion.1,3
Other approaches to strengthen T-cell functioning involve the use of LAG3—considered the third immune checkpoint inhibitor after CTLA-4 and PD-1. It functions differently from other checkpoints, as it is rapidly shed by ADAM metalloproteases and is unusual in being an obligate dimer. In March 2022, the FDA approved a fixed-dose combination of relatlimab (Opdualog; Bristol Myers Squibb) with nivolumab (Opdivo; Bristol Myers Squibb) for the treatment of metastatic melanoma, marking a major milestone in LAG3-targeted immunotherapy.1
The dual blockade of PD-1 and LAG3 leads to distinct changes in CD8+ T cells, including enhanced T-cell signaling and partial reversal of exhaustion. Although some exhaustion pathways remain, the overall profile suggests improved immune activation and durability of response compared with PD-1 inhibition alone.1
"Our data suggested that PD-1 generates sprinters that function effectively in a short period of time, perhaps [without] the durability required for a long-term fight against cancer, whereas this PD-1 LAG3 combination produced marathon runners that had durability, giving rise to increased efficacy over time,” said Dario Vignali, PhD, from the University of Pittsburgh.1
The evolving understanding of the TME continues to reshape approaches to cancer immunotherapy. As research progresses, the ability to manipulate the immune microenvironment may be key to overcoming resistance and enhancing immunotherapy outcomes.
REFERENCES
1. Varner J, Minn A, Vignali D. One step ahead: Preventing tumor adaptation to immunotherapy. 2025 ASCO Annual Meeting. May 30, 2025, to June 3, 2025.
2. Gerlach A. Targeting resistance: Navigating the complexity of the tumor microenvironment and CAR T-cell efficacy. Pharmacy Times. August 16, 2024. Accessed May 31, 2025. https://www.pharmacytimes.com/view/targeting-resistance-navigating-the-complexity-of-the-tumor-microenvironment-and-car-t-cell-efficacy
3. Mathew D, Marmarelis ME, Foley C, et al. Combined JAK inhibition and PD-1 immunotherapy for non-small cell lung cancer patients. Science. 2024;384(6702):eadf1329. doi:10.1126/science.adf1329
Newsletter
Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights.