News
Article
FDA Approves Dupilumab, Marking First Targeted Therapy in a Decade for Chronic Spontaneous Urticaria
Author(s):
Key Takeaways
- Dupilumab (Dupixent) is now FDA approved for CSU in patients 12 years and older who are unresponsive to H1 antihistamines.
- This approval marks the first new CSU treatment in over a decade, offering new management options.
Multiple phase 3 clinical trials demonstrated dupilumab’s (Dupixent; Regeneron, Sanofi) effectiveness in reducing hives and itch in patients with chronic spontaneous urticaria compared with placebo.
The FDA has granted regulatory approval to dupilumab (Dupixent; Regeneron, Sanofi) for the treatment of adults and adolescents 12 years and older with chronic spontaneous urticaria (CSU) who remain symptomatic following histamine-1 (H1) antihistamine treatment, according to a news release from Regeneron and Sanofi. This marks the first new treatment approved for CSU in over 10 years.1
Image Credit: © Aleksej - stock.adobe.com
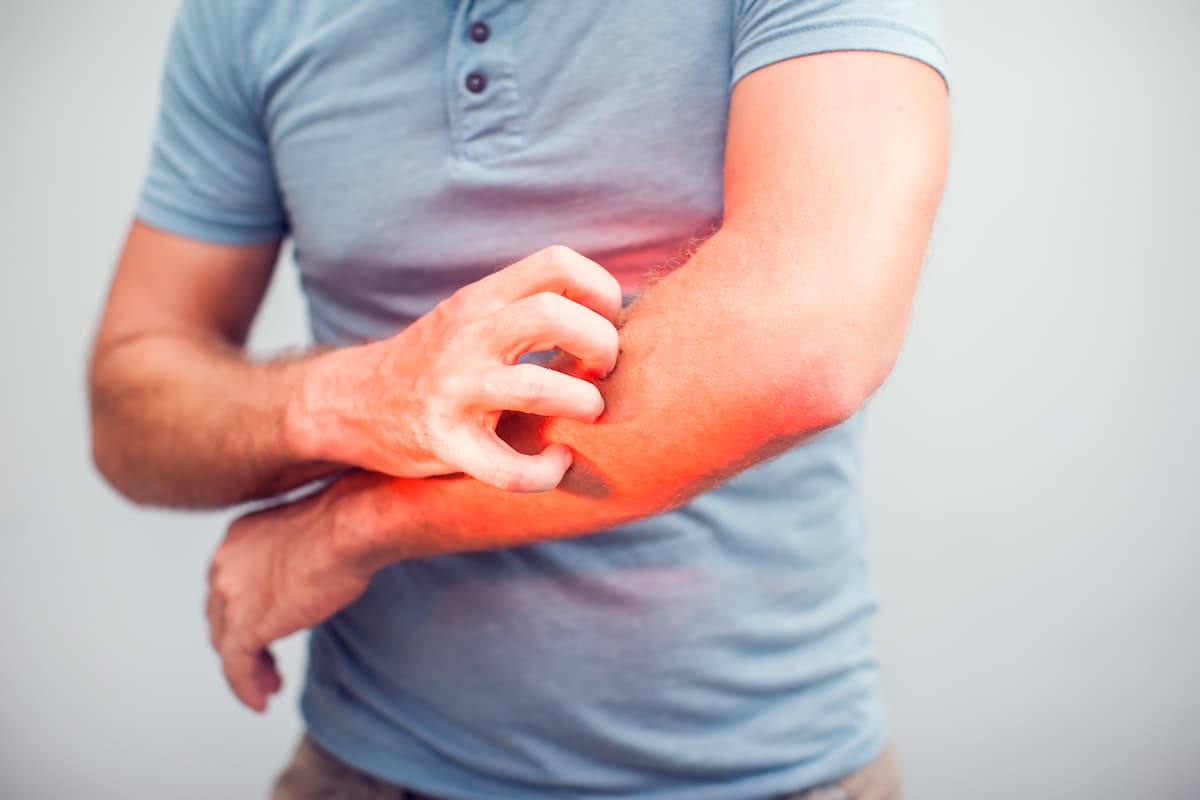
“People with chronic spontaneous urticaria experience sudden, unpredictable hives and severe itch that cause a significant, and often overwhelming, burden on their everyday lives,” Kenneth Mendez, president and CEO of the Asthma and Allergy Foundation of America, said in the news release. “The approval of this treatment offers patients more options and the chance to control their disease.”1
Dupilumab Reduces Hives, Itch Activity
In 2 phase 3 clinical trials—Study A and Study C—dupilumab demonstrated effectiveness as an add-on treatment to standard-of-care antihistamines in biologic-naive, symptomatic patients with CSU compared with treatment using antihistamines alone. In each trial, key primary and secondary end points with dupilumab were met, with investigators observing significant reductions in both urticaria activity—which is a composite of hives and itch—and itch severity. Additionally, treatment with dupilumab heightened the chances of well-controlled disease or complete response compared with placebo at 24 weeks of follow-up, according to the news release.2,3
Dupilumab demonstrated additional critical safety and tolerability data in Study B, a phase 3, randomized, placebo-controlled clinical trial. In the trial, the proportions of patients with any treatment-emergent adverse events (TEAEs) were similar between those treated with dupilumab and placebo. Importantly, only 5 patients (4.0%) treated with dupilumab in the total cohort (n = 124) developed serious TEAEs. When pooling safety data from all 3 trials, investigators observed a tolerable and positive profile of AEs, with the most common AE in patients treated with dupilumab being injection site reactions.1,4
By inhibiting the signaling of IL-4 and IL-13 pathways, which are 2 major drivers of inflammation, dupilumab helps suppress the symptoms of CSU and reduce severe itching. Previously, dupilumab has received indications for atopic dermatitis, asthma, chronic obstructive pulmonary disease, and other allergic conditions.5
A New Option for Patients, Pharmacists
CSU can afflict patients with unpredictable and intense episodes of hives and itching, which can severely burden them and impact their daily routines. With the approval of dupilumab for this condition, patients will have a new option for treating their daily symptoms for the first time in a decade.1,5
“CSU patients with uncontrolled disease experience highly burdensome itch and hives that can significantly disrupt daily living,” Alyssa Johnsen, MD, PhD, global therapeutic area head, immunology and oncology development at Sanofi, said in the news release. “This FDA approval provides a new treatment option to help address the underlying drivers of these severe and recurring signs and symptoms.”1
Pharmacists stand to play a critical role in prescribing dupilumab to patients with CSU at optimal dosage and timeframe, and can participate in patient counseling among a multidisciplinary care team to educate patients on what to expect with dupilumab treatment. Additionally, they will remain essential in managing flare-ups of CSU and any TEAEs that may emerge with dupilumab administration.
REFERENCES
1. Regeneron. Dupixent (dupilumab) approved in the U.S. as the first new targeted therapy in over a decade for chronic spontaneous urticaria (CSU). News Release. Released April 18, 2025. Accessed April 18, 2025.
2. Regeneron. Late-breaking phase 3 data at 2022 AAAAI Annual Meeting show Dupixent (dupilumab) significantly reduced itch and hives in patients with chronic spontaneous urticaria. News Release. Released February 26, 2022. Accessed April 18, 2025. https://investor.regeneron.com/news-releases/news-release-details/late-breaking-phase-3-data-2022-aaaai-annual-meeting-show
3. Regeneron. Dupixent (dupilumab) late-breaking positive phase 3 data in chronic spontaneous urticaria to be presented at ACAAI. News Release. Released October 24, 2024. Accessed April 18, 2025. https://newsroom.regeneron.com/news-releases/news-release-details/dupixentr-dupilumab-late-breaking-positive-phase-3-data-chronic
4. Maurer M, Casale TB, Saini SS, et al. Dupilumab in patients with chronic spontaneous urticaria (LIBERTY-CSU CUPID): Two randomized, double-blind, placebo-controlled, phase 3 trials. Journ Allergy Clin Immunology. 2024;154(1):184-194. doi:10.1016/j.jaci.2024.01.028
5. McGovern G. Dupilumab shows significant reductions in itch and hive activity in patients with CSU. Pharmacy Times. Published November 5, 2024. Accessed April 18, 2025. https://www.pharmacytimes.com/view/dupilumab-shows-significant-reductions-in-itch-and-hive-activity-in-patients-with-csu
Newsletter
Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights.