Article
Daily Medication Pearl: Risankizumab-rzaa (Skyrizi) for Plaque Psoriasis
Author(s):
Risankizumab-rzaa treats moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Medication Pearl of the Day: Risankizumab-rzaa (Skyrizi)
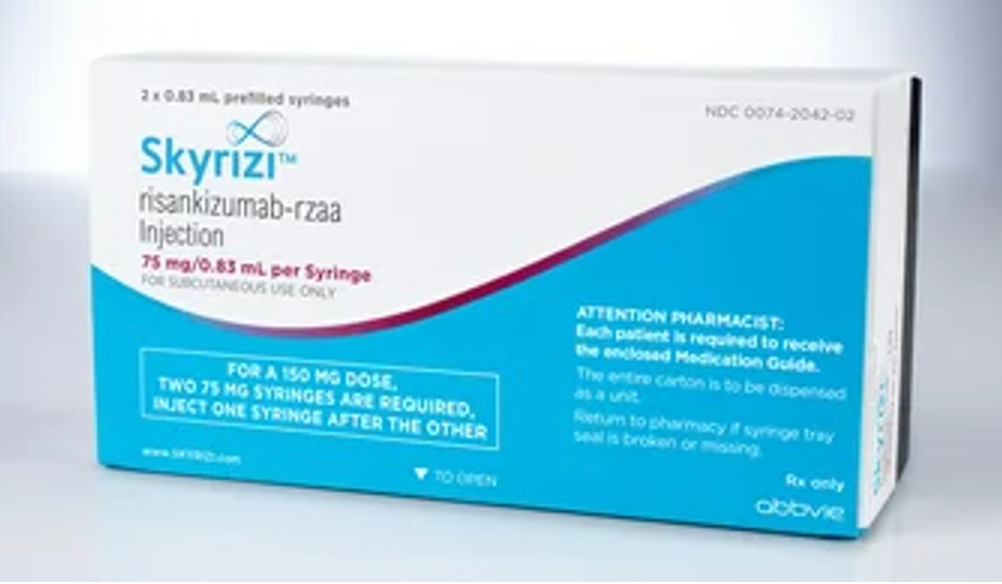
Indication: Risankizumab-rzaa is an interleukin (IL)-23 antagonist indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Insight:
- Dosing: 150 mg administered by subcutaneous injection at week 0, week 4, and every 12 weeks thereafter.
- Dosage form: Injection 150 mg/mL in each single-dose prefilled pen. Injection 150 mg/mL in each single-dose prefilled syringe. Injection 75 mg/0.83 mL in each single-dose prefilled syringe.
- Adverse events (AEs): Most common AEs (≥ 1%) are upper respiratory infections, headache, fatigue, injection site reactions, and tinea infections.
- Mechanism of action: Risankizumab-rzaa is a humanized immunoglobulin G1 (IgG1) monoclonal antibody that selectively binds to the p19 subunit of the IL-23 cytokine and inhibits its interaction with the IL-23 receptor. IL-23 is a naturally occurring cytokine that is involved in inflammatory and immune responses.
- Manufacturer: Abbvie
Sources:
Newsletter
Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights.






